Scientists in Burkina Faso have deployed a new weapon in the fight against malaria, and waded into a thorny bioethics debate, by letting loose thousands of genetically sterilised mosquitoes.
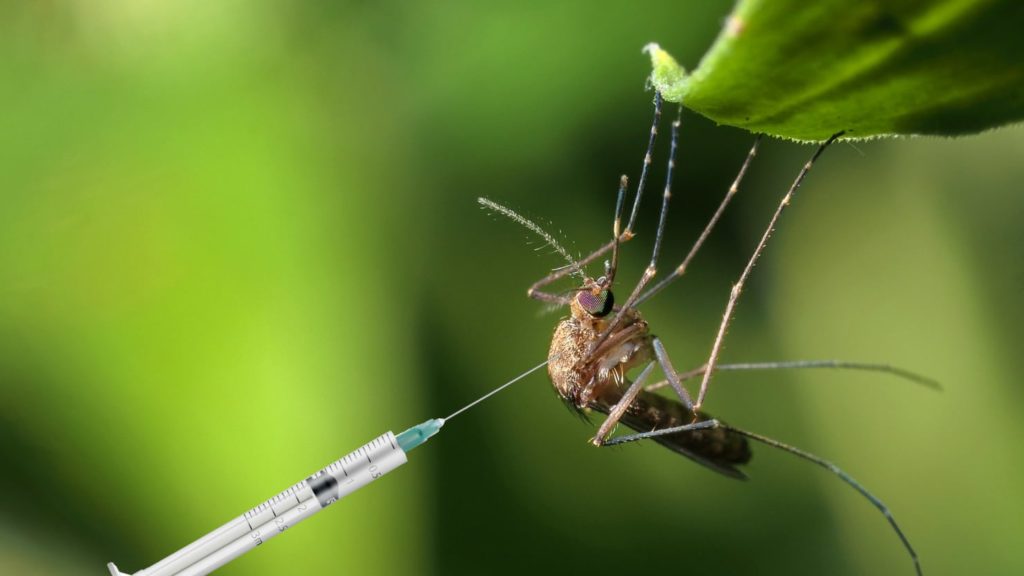
Their experiment is the first outside the lab to release genetically altered mosquitoes in the hope of reducing their ability to spread the often-deadly disease.
It works using a technique called a gene drive, which edits and then propagates a gene in a population – in this case to prevent males from producing offspring.
Investments in anti-malarial drugs, mosquito nets and insecticides have slowed malaria over the past two decades in Africa, which accounts for more than 90 per cent of global cases.
But malaria still killed more than 400,000 people across the continent in 2017, and the WHO says progress against the disease is stalling, leading researchers to push for fresh approaches.
“The conventional tools that we have at our disposal today have reached their limit,” said Dr Abdoulaye Diabate, who is running the experiment for Target Malaria, a research consortium backed by the Bill & Melinda Gates Foundation.
One hot evening in July, Diabate’s researchers peeled off mesh nettings from wire-rimmed containers to release about 5,000 male mosquitoes into Souroukoudinga, a village in western Burkina Faso.
The mosquitoes had been injected as embryos with an enzyme that sterilises them.
“Our objective is not to eradicate mosquitoes,” said Diabate, noting the enzyme targets only the three main species – out of more than 3,500 worldwide – that carry malaria.
“The objective is… to reduce the density of these mosquitoes.”
Target Malaria is also developing an enzyme preventing male mosquitoes from passing on X chromosomes.
This results in male offspring, reducing malaria since only female mosquitoes bite – males mostly feed off plant honeydew.
Diabate said he hoped the new approaches would win approval from national regulators in the coming years for widespread use.
Using a gene drive proved effective in lab experiments at Imperial College London, where researchers last year said they had succeeded in wiping out populations of caged mosquitoes within 11 generations.
Activists in Burkina fear unintended environmental consequences.
They point to Burkina’s experiment with genetically modified cotton a few years ago, which farmers said had lowered quality and was ultimately abandoned in favour of conventional seeds.
“We are not going to allow Burkinabes to be used as guinea pigs,” said Ali Tapsoba, a Burkinabe activist.
“If we intoxicate one link in the food chain, we are going to intoxicate the next link.”
Those concerns echo beyond Burkina.
Last November, signatories of a UN convention on biodiversity noted “uncertainties regarding engineered gene drives’’.
Critics of gene drives fear they could be used to manipulate human genetics or develop a bioweapon.
Researchers in Brazil have also released genetically modified mosquitoes to control diseases like yellow fever and Zika, but it is not clear how effective that has been.
Target Malaria says it consults with communities and that research is overseen by national regulatory authorities and an independent ethics committee.
Two months after the mosquitoes were released, Souroukoudinga chief Pascal Traore said villagers were happy with the experiment’s progress.
“We all believe that the project could reduce the malaria that kills our sons and daughters,” he said.
“This project is not just for us, but for the entire world.”
